

Today we’d like to introduce you to Bill Gruber.
Bill, please share your story with us. How did you get to where you are today?
Following Graduation from Washington State University I joined Procter and Gamble as a sales representative selling laundry and dish detergent. After five years I was hired by Boston Scientific to be a sales representative with their peripheral vascular division. After 10 years of working my way up to the position of Director of Atherosclerotic Diseases, I left to join a troubled startup in the spinal products space (Cortek) as the VP of Sales and Marketing.
When Cortek sold I briefly worked as President of Hemedex in Cambridge, MA before joining Spray Venture Partners in Newton, MA as an entrepreneur-in-residence. Along with the folks at Spray Ventures we founded Interlace Medical Corporation where I was President and CEO. While at Interlace, we developed a surgical product for removing fibroids in women with abnormal uterine bleeding. Interlace sold to Hologic in Marlborough, MA in 2011. Later that year I was asked to join Solace Therapeutics which was a perennial startup focused developing a device for women with stress urinary incontinence. As the Company’s President and CEO, I lead a team of 18 extremely talented engineers, clinical and operations people focused on bringing this life changing technology to market. Like many of us, my career path from soap to incontinence is a circuitous path and frequently defied logic. However, I’m happy with the decisions I have made and remain focused on developing and delivering outstanding medical technology to solve vexing problems while providing a great financial return to investors.
Great, so let’s dig a little deeper into the story – has it been an easy path overall and if not, what were the challenges you’ve had to overcome?
Transitioning from selling soap to grocery stores to teaching doctors procedures in the operating room was a huge transition. The training at Boston Scientific was difficult and rigorous. It was a medical boot camp but once complete, gave me the ability to make the transition to the medical device industry where that learning continued and I can’t imagine doing anything else other than developing medical devices and building medical device companies.
Managing medical device companies is all about problem solving and decision making. We hire people who are skilled at doing both, as every day is filled with challenges.
In the medical device field you are forced to work with a bureaucratic regulatory system that moves at a very slow pace while managing a group of type A team members chomping at the bit to make new technology available.
More recently, trying to attract investors to invest money in medical device companies has become more difficult as the venture capital industry continues to change.
Running these companies can best be described as the circus performing spinning a dozen plates on the top of six foot poles without letting any topple off.
Solace Therapeutics – what should we know? What do you guys do best? What sets you apart from the competition?
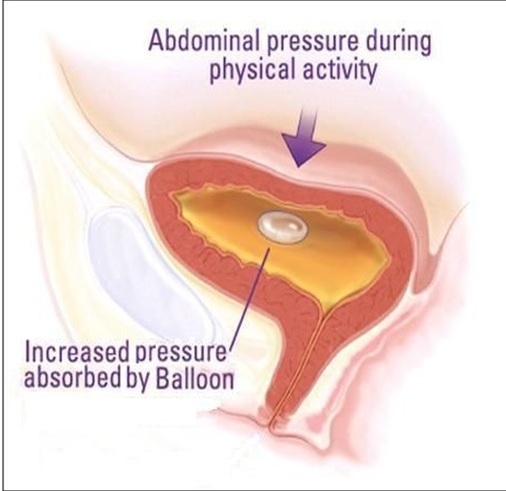
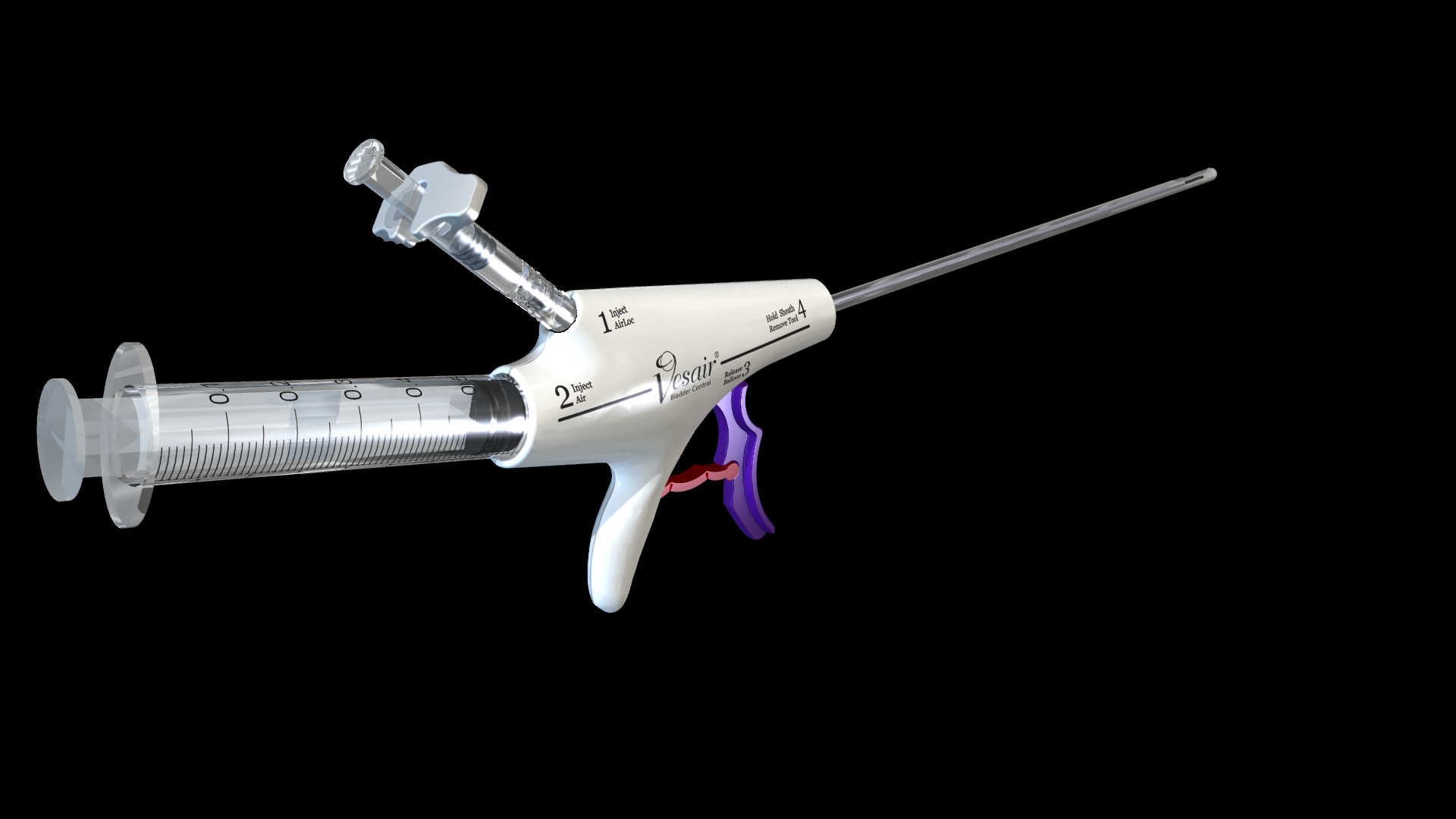
Solace Therapeutics has developed a balloon that is placed into a woman’s bladder to act as a shock absorber during moments of stress such as laughing, coughing, sneezing or lifting that cause urine leakage. The balloon is designed to be placed in a doctor’s office without the need for anesthesia and works instantly providing relief from incontinence. The balloon remains in place floating passively at the top of the bladder for up to one year. On an annual basis the woman will go back to her doctor who will remove and replace the balloon for up to another 12 months. The device is not yet approved in the US and requires the Company to conduct a pivotal trial in support of an FDA submission for approval. This technology is very exciting because over 15 million US women have this condition and the options for treatment are very few.
What moment in your career do you look back most fondly on?
Creating Interlace Medical which developed a device for removing fibroids from inside the uterus to help women with abnormal uterine bleeding has been my proudest moment. The market for these devices was approximately $10 million when we launched the device. Today the Myosure Myomectomy System is the standard of care and the market is over $240 million with thousands and thousands of women now able to avoid a hysterectomy with a safer, uterine-sparing procedure that is performed on an outpatient basis.
Contact Info:
- Address: 135 Newbury St
Framingham, MA 01701 - Website: www.solacetx.com
- Phone: 508-283-1200





Getting in touch: BostonVoyager is built on recommendations from the community; it’s how we uncover hidden gems, so if you know someone who deserves recognition please let us know here.
















